Search Posts
Recent Posts
- Put Your Money Where Your Mouth Is October 6, 2024
- Sign on the Dotted Line September 26, 2024
- Happy Anniversary Grampa John September 25, 2024
- The Two Percent September 5, 2024
- Cancel Culture August 25, 2024
Categories
Just Dew It!
Over a decade ago, I worked in the BP Gulf Oil Spill. I held several different positions over the two- and one-half years I was there. For this piece, I refer to one particular position: safety rep for the beach cleanup.
During my time there, I witnessed and responded to several heat stress and heat stroke victims. Upon speaking with them, all of them swore they had been hydrating sufficiently and about 90 percent of them had been drinking Mt. Dew. A responder in the field told me that Mt Dew (although a consumable drink) was by no means a method of hydration. In fact, it would take multiple servings of the same quantity of water to return someone’s body to the level of hydration they were at prior to consuming Mt. Dew.
I have consumed my fair share of soda in my lifetime although never Mt. Dew. I consume less now. But I have always wondered about what I was told that day. Even more to the point, I wondered about our Food and Drug Administration.
In looking up information regarding this on the web, I found the following excerpts of information in various locations:
(Valuates, 2024)
Brominated vegetable oil (BVO) is a complex mixture of plant-derived triglycerides that have been modified by atoms of the element bromine bonded to the fat molecules. Brominated vegetable oil is used to help emulsify citrus-flavored soft drinks, preventing them from separating during distribution. Brominated vegetable oil has been used by the soft drink industry since 1931, generally at a level of about 8 ppm.
In the United States, BVO was designated in 1958 as generally recognized as safe (GRAS),[2] but this was withdrawn by the U.S. Food and Drug Administration (FDA) in 1970. (Wikipedia, 2024)
In 2016, PepsiCo pledged to remove brominated vegetable oil from all their products, but for years beyond that, Mountain Dew, Gatorade, and MTN DEW Amp still contained the ingredient. (Center for Science in the Public Interest, Jul 2, 2024)
In October 2023, California Governor Gavin Newsom approved a law that banned the manufacture, sale, and distribution of brominated vegetable oil (along with three other additives: potassium bromate, propylparaben, and Red 3). This was the first law in the U.S. to ban it.[6] The ban of its use in foods will go into effect in 2027. (Wikipedia, 2024)
In 2024, the FDA revoked regulations allowing the use of BVO in food.[5] The agency concluded that the intended use of BVO in food is no longer considered safe after the results of studies conducted in collaboration with the National Institutes of Health (NIH) found the potential for adverse health effects in humans. (Wikipedia, 2024)
Between 2012 and 2019, the FDA’s product recall classifications are significantly influenced by firms’ lobbying activities. Lobbying firms appear to have received more favorable (i.e., less severe) recall classifications compared to non-lobbying firms. (NIH National Library of Medicine, 2024)
So how much has PepsiCo spent on lobbying? Well, to give you some idea, in 2021 PepsiCo’s expenditures for lobbying reached $3,060,000. (Open Secrets, 2024
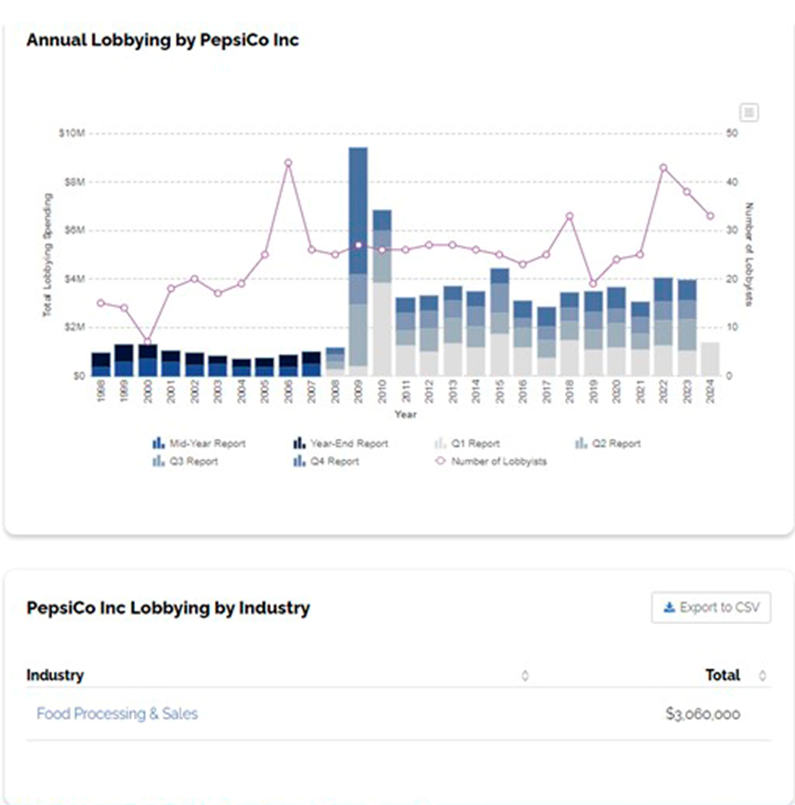
In the years 2009 and 2010, funds spent lobbying more than doubled that of the $3,060,000 spent in 2021.
Now that the FDA’s proposed rule is finalized, consumers will soon see changes to their favorite orange, lemon-lime, or pineapple sodas. The rule is effective on August 2, 2024, and manufacturers have one year from that date to “reformulate, relabel, and deplete the inventory” of their BVO-containing products. (Center for Science in the Public Interest, 2024)
I’m no scientist. But if what I have found is true, it has taken the FDA approximately 50 years to remove a harmful substance from a daily consumable sold in the United States. This is an agency whose sole purpose is to ensure the safety of the citizens of the United States.
The Food and Drug Administration is the oldest comprehensive consumer protection agency in the U. S. federal government. Since 1848 the federal government has used chemical analysis to monitor the safety of agricultural products — a responsibility inherited by the Department of Agriculture in 1862 and later by the FDA. (US Food & Drug Administration, 2024)
It gets worse. The following is from an article by the Environmental Health News.
In the U.S., approximately 10,000 chemicals can be purposely added to food or enter the food supply through processing equipment and packaging, and 60 percent of the calories ingested are from ultra-processed foods. In 1958, Congress gave the FDA authority to regulate chemicals intentionally added to food or to food contact materials, commonly known as food additives, to ensure their use is safe.
We wanted to investigate whether and how food manufacturers and the FDA had implemented the cumulative effect requirement. To do that, we downloaded and reviewed all 877 safety determinations contained in the Generally Recognized as Safe (GRAS) notifications inventory. These notices were voluntarily submitted by food manufacturers to the FDA between 1997, when GRAS notification program began, and March 24, 2020. We looked at GRAS notices because they are publicly available and FDA rules require that food manufacturers include in the notice an explanation of how they considered the cumulative health effect of a new additive. Unfortunately, our investigation showed that both the FDA and the food manufacturers appeared to have ignored this crucial safety requirement.
We used the agency’s online research tool and identified twenty-one documents related to food chemicals. For each document, we searched for key terms including “cumulative effect”, “chemically related”, “pharmacological effects”, and “pharmacologically related”. We also searched for references to key regulations or statutory provisions directly related to the cumulative effect requirement. We found next to nothing and what information was there was either incomplete or confusing.
Ten documents did not mention the legal requirement and two simply restated it. Four documents created confusion by using terms such as ‘cumulative exposure’ or ‘cumulative intake.’ Five documents provided incomplete and potentially misleading information (Environmental Health News, 2024)
I intend to have more on this in the days to come.
Get ready to R-O-A-R!!!
References:
Center for Science in the Public Interest, retrieved from the world wide web on July 14, 2024from https://www.cspinet.org/cspi-news/bvo-fda-finally-bans-brominated-vegetable-oil
Environmental Health News, retrieved from the world wide web on July 14, 2024 from https://www.ehn.org/health-issues-associated-with-food-additives-2649620272.html?gad_source=1&gclid=EAIaIQobChMI7eOduPWmhwMV_zHUAR2-AgqkEAAYAiAAEgIFEvD_BwE
National Library of Medicine, retrieved from the world wide web on July 14, 2024 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10280948/
Open Secrets, retrieved from the world wide web on July 14, 2024 from https://www.opensecrets.org/federal-lobbying/clients/summary?cycle=2021&id=D000000200
US Food and Drug Administration, retrieved from the world wide web on July 14, 2024 from https://www.fda.gov/about-fda/fda-history
Valuates Reports, retrieved from the world wide web on July 14, 2024 from https://reports.valuates.com/market-reports/QYRE-Auto-10S14636/global-brominated-vegetable-oil
Wikipedia, retrieved from the world wide web on July 14, 2024 from https://en.wikipedia.org/wiki/Brominated_vegetable_oil#:~:text=Regulation%20and%20use-,United%20States,use%20of%20BVO%20in%20food.